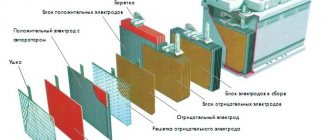
Why is electrolyte needed in a battery?
Lead-acid batteries undergo electrochemical processes that produce electricity. These processes are possible only with the direct participation of the electrolyte.
The battery has negatively and positively charged plates. They contain lead conductive elements. They may have various additives, which determine the type of battery.
The degree of charge of the battery depends on the density of the electrolyte. If it is increased, the battery will be overcharged, and an aggressive environment can generally harm the battery. When it is lowered, the battery will be discharged. The density of concentrated battery acid is 1.835. In order to bring the value to normal, it is necessary to use distilled water.
The range of indicators for a normally charged battery is within 1.23 – 1.25 g/cm3. When the engine starts, the audio and light elements are turned on, the battery discharges. This happens due to the occurrence of an electrochemical reaction. The substance loses sulfuric acid, resulting in a drop in density and discharge of the battery.
Electrolyte
The electrolyte in the battery has run out
Almost every process in the battery depends on the electrolyte - this is the liquid in which all the chemical processes of the battery take place and electricity is generated. It consists of a mixture of acid and distilled water (approximate ratio of 35% to 65%).
First of all, it is necessary to maintain the level of the solution inside the battery:
Find out the charging time of your battery
- If the level is below normal, this will lead to exposure of the battery plates and shedding of the battery plates.
- When the level is higher, this is also bad. In the summer, this may not be very noticeable, but in the winter, due to the increased level, the electrolyte can freeze, and not just freeze, but completely “break” the battery case.
We can draw a logical conclusion that maintaining the optimal level of electrolyte in a car battery is an immediate necessity.
How much electrolyte should be in the battery
The car owner must constantly monitor the electrolyte level. If it drops, you will need to add distilled water. Depending on the battery capacity, the volume of the mixture will depend:
- 55 Ah – 2.5 l;
- 60 Ah – 2.7-3 l;
- 62 Ah – about 3 l;
- 65 Ah – about 3.5 l;
- 75 Ah – 3.7-4 l;
- 90 Ah – 4.4-4.8 l;
- 190 Ah – about 10 liters.
Depending on the manufacturer, technology and model, these figures may vary, so they are conditional. The main thing to remember is that the electrolyte must completely cover the plates by 10-15 mm and in no case should they protrude.
Battery cover sensor
Electrolyte volume in batteries 55 and 60 Ah
The capacity of the battery varies; the energy storage device can be designed for either 35 or 230 Ampere-hours. But low-capacity car batteries (from 35 to 45 A/h) are rarely used in practice; they do not provide the required starting current and drain faster from the load. The most common standard for passenger cars is 55 and 60 Ampere-hours; batteries of these types are usually used on cars with engines up to 2 liters, with any type of transmission and a small amount of additional equipment.
To fully refill a “55” battery, approximately two and a half liters of electrolyte are required; depending on the type and manufacturer, the weight of the battery may vary slightly, but on average it is 15 kilograms. An empty 60 Ah battery should be filled with 2.7 to 3 liters of sulfur solution (H2SO4), the mass of a charged and ready-to-use energy storage device is approximately 17-18 kg.
How to check the electrolyte level in a battery
Most batteries on the banks have a scale with MIN and MAX values, and it is in this range that the electrolyte should be. There are models with plastic tabs under the stoppers that go down inside the cans; they should be immersed in the liquid by 5 mm.
Modern batteries are equipped with a special sensor on the case, which indicates a low electrolyte level and the degree of discharge of the battery.
If for some reason there are no symbols, then you can resort to the following method:
- Take a small, clean tube;
- Wipe the battery case clean and dry;
- Unscrew the lids from all the cans;
- We lower the tube into the jar at a right angle and touch its plates;
- Press the top hole of the tube tightly with your finger;
- Carefully pull out and measure the height of the liquid (should be at the level of 10-15 mm);
- We repeat the procedure with all banks.
If there is not enough mixture in the jars, you will need to add distilled water. You need to top up until the plates are completely closed. Distilled water can be purchased at a pharmacy. You cannot use regular tap water.
If you operate a car with “bare plates,” they will quickly crumble and fall apart.
There are now maintenance-free batteries on the market. If there are no lids on the body, then you will not be able to add water into it.
How to check battery density
To ensure proper operation of the battery, the density of the electrolyte should be checked every 15-20 thousand kilometers. Measuring the density in a battery is carried out using a device such as a densimeter. The device of this device consists of a glass tube, inside of which is a hydrometer, and at the ends there is a rubber tip on one side and a bulb on the other. To check, you will need to: open the cap of the battery can, immerse it in the solution, and use a bulb to draw in a small amount of electrolyte. A floating hydrometer with a scale will show all the necessary information. We will look in more detail at how to properly check the battery density below, since there is also a type of battery called maintenance-free, and the procedure for them is somewhat different - you do not need absolutely any devices.
Density indicator on a maintenance-free battery
The density of a maintenance-free battery is displayed by a color indicator in a special window. The green indicator indicates that everything is normal (the degree of charge is between 65 and 100%), if the density has dropped and recharging is required, the indicator will be black. When a white or red light is displayed in the window, an urgent addition of distilled water is needed. But, however, the exact information about the meaning of a particular color in the window is on the battery sticker.
Now we continue to further understand how to check the electrolyte density of a conventional acid battery at home.
Checking the electrolyte density in the battery
So, in order to be able to correctly check the density of the electrolyte in the battery, first of all we check the level and, if necessary, adjust it. Then we charge the battery and only then start checking, but not immediately, but after a couple of hours of rest, since immediately after charging or adding water there will be unreliable data.
It should be remembered that density directly depends on air temperature, so check the correction table discussed above. After taking liquid from the battery can, hold the device at eye level - the hydrometer should be at rest, floating in the liquid without touching the walls. Measurements are taken in each compartment, and all indicators are recorded.
Table for determining battery charge based on electrolyte density.
“>
What can you add water or electrolyte to the battery?
If the battery plates are not closed, it means that the level of the substance is not high enough and you need to add distilled water. At the same time, you cannot fill in plain water, since it contains various impurities that can disrupt the operation of the battery and cause it to fail.
If the mixture has sufficient density, then distilled water is added. If the density of the electrolyte approaches the lower mark, then you can add an alkaline mixture. When current passes through the battery, acid is consumed. The process is called diffusion of H2SO4 between the volume and the electrode. This maintains voltage at the battery terminals.
Electrolyte and its role in the battery
So, let's start by defining what is called an electrolyte. It is simply a solution of sulfuric acid and distilled water. Moreover, the presence of any foreign impurities is unacceptable, because then its density will change, which will most negatively affect the operation of the battery. Its level also plays a very important role. So, for example, if it is below normal, the internal plates will dry out and the power of the car battery will decrease.
You should not think that the solution will be to add more liquid than normal, because in this case the acid will corrode the outer part of the unit. In addition, you may encounter problems such as rapid self-discharge of the battery or failure of the voltage regulator. In general, the proper level of electrolyte is the key to the normal functioning of your car, so you should periodically inspect the battery.
What to do if your electrolyte level is low
If the electrolyte level drops, it will need to be topped up. First you need to fill it with distilled water and put the battery on charge. If the density does not begin to increase, you can try draining all the liquid and completely replacing the electrolyte.
Sometimes the battery is discharged to such an extent that adding new mixture may not be practical. If the element did not last long, then this procedure can be done. But this doesn't always work on old batteries. They become unusable if operated on an electrolyte with a low alkaline level.
Still have questions about your electrolyte level or have something to add? Then write to us about it in the comments, this will make the material more useful, complete and accurate.
What to do if the electrolyte solution level is low or high
The motorist will be able to restore the volume of the acid solution independently, subject to safety precautions and the availability of materials and tools. Let us next consider whether it is possible to add purchased electrolyte to a car battery yourself. Let's determine how to properly restore the balance of liquid in jars.
For topping up, motorists can use both ready-made electrolyte and distillate. However, most often the second option is necessary, since during operation and charging periods, water mostly boils away, and the acid concentration increases. Adding the electrolyte directly is relevant for those cases when it splashed out of the common container or the solution was lost through cracks or microcracks.
Having identified an excess of liquid level, it is necessary to select the excess. For this operation, a rubber bulb with a hard tip inserted, for example from a glass tube, is suitable. After taking the liquid, we repeat the measurement and restore the balance.
It is strictly unacceptable to turn the battery over to drain excess acid solution, as there is a risk of destruction of the fragile lead plates. This applies to batteries with a long service life.